How Stable Packaging Helps Medicines Survive Africa’s Heat
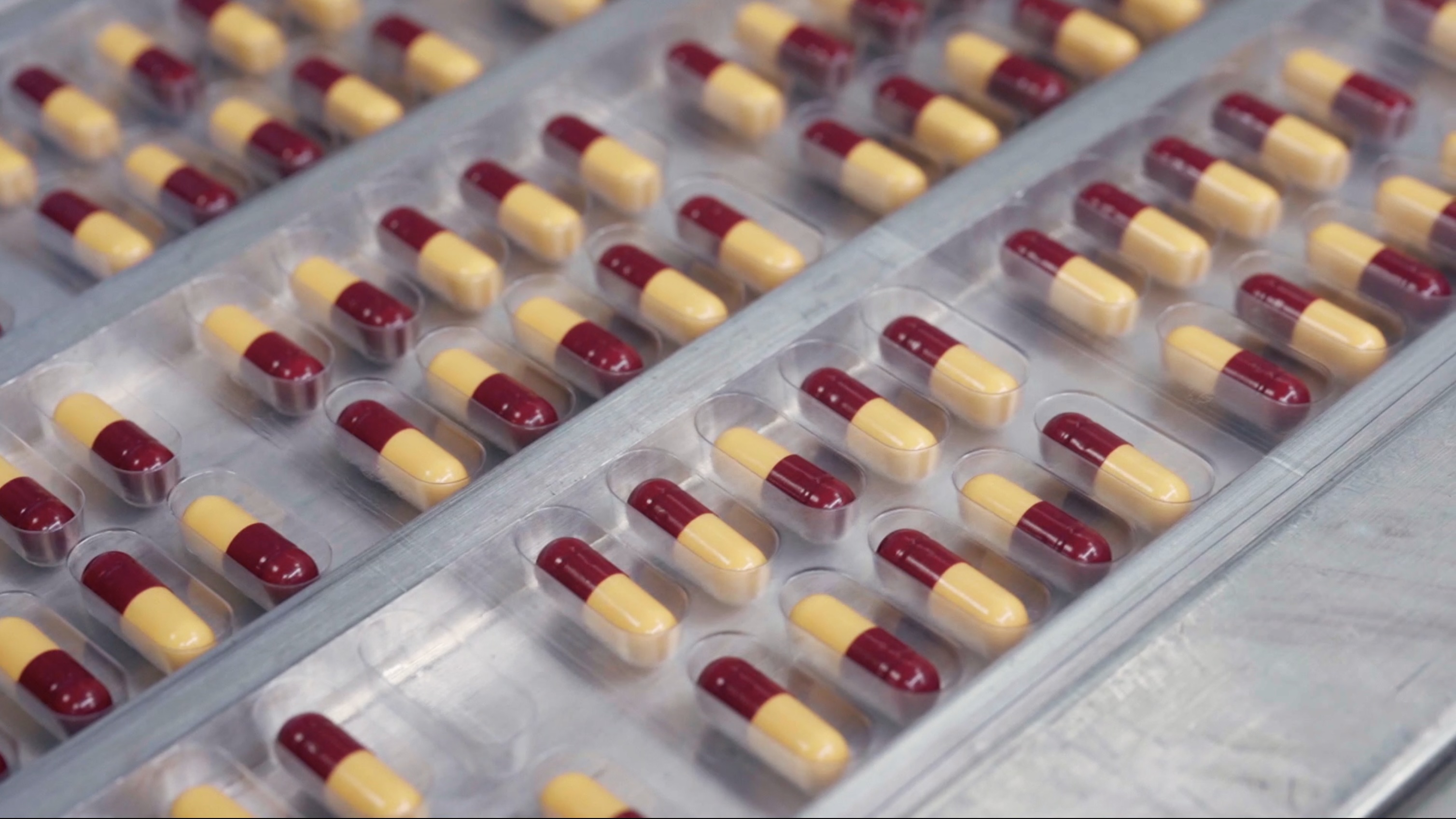
Across many African countries, pharmaceutical products are routinely exposed to high temperatures, intense humidity, and long supply chain routes. For sensitive medicines packaging quality can determine whether a product reaches patients in its intended state.
At Riva Pharma, packaging is treated as a core part of product integrity. This article focuses on tablets and capsules, which are particularly sensitive to heat and humidity. The goal is simple: ensure every capsule or tablet remains potent, safe, and stable from factory to pharmacy.
1. Why Climate Stability Is Critical
Medicine can degrade faster when exposed to heat, moisture, oxygen and direct sunlight. If stability is compromised, the medicine may lose potency, become less effective. In regions where pharmacies and clinics may lack climate-controlled storage, packaging must compensate for environmental challenges.
2. Africa’s Real-World Supply Chain Conditions
Transport and storage challenges include:
- long road networks
- non-refrigerated containers
- delays at ports and borders
- rural clinics without air-conditioning
- seasonal humidity (up to 90% in some regions)
This means packaging must protect medicines through the worst-case scenario.
3. Protective Packaging Used in Our Products
At Riva Pharma, several layers of barrier protection are used:
A. High-Barrier Blister Packs
- Alu-Alu (Aluminum-Aluminum) for maximum protection
- PVC/PVDC for high moisture resistance
These blisters significantly delay moisture penetration, even in humid climates.
B. Foil Seals and Laminated Films
Heat-sealed foil improves oxygen and moisture barriers, reducing degradation risk.
C. Light-Resistant Cartons
Many beta-lactams are photosensitive; secondary packaging prevents light exposure and adds physical protection during transport.
4. Stability Testing for Climate Zone IVb
African markets generally fall under WHO Climate Zone IVb, representing:
- 30°C ± 2°C
- 75% ± 5% humidity
Products at RIva Pharma Pharmaceuticals undergo:
- Accelerated stability studies
- Long-term stability testing under IVb conditions
- Packaging integrity validation
This ensures that medicines maintain their potency throughout distribution.
5. Protecting Product Integrity During Transport
In addition to packaging design, we collaborate with distributors on:
- correct pallet wrapping
- avoiding exposure to direct sunlight
- using well-ventilated storage
- monitoring container temperature during sea shipment
When packaging and handling align, medicines maintain optimal quality across the entire supply chain.
Conclusion
In many African regions, strong and stable packaging is essential. It keeps medicines effective, protects patients, and helps healthcare providers trust the products they use every day. This is why Riva Pharma focuses on reliable materials and strict quality checks to make sure every capsule, tablet, and vial stays safe from our factory until it reaches the patient.
Ready to Connect?
Whether you're a distributor, healthcare provider, or investor — we’d love to hear from you.
